You are here
NIH panel says plasma should not yet be considered standard care for cornonavirus
Primary tabs
NIH panel says plasma should not yet be considered standard care for cornonavirus
Wed, 2020-09-02 01:18 — mike kraft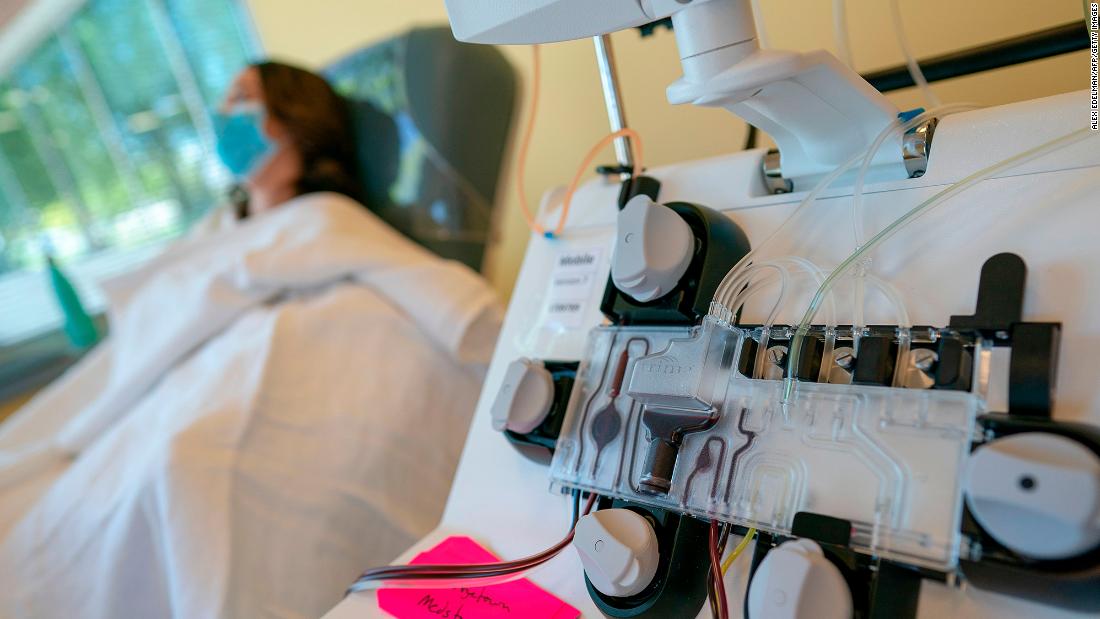 Plasma should not be considered standard care for coronavirus, NIH panel says Convalescent plasma taken from Covid-19 survivors should not be considered a standard of care for coronavirus, an NIH panel says. CNN
Plasma should not be considered standard care for coronavirus, NIH panel says Convalescent plasma taken from Covid-19 survivors should not be considered a standard of care for coronavirus, an NIH panel says. CNN CNN)A National Institutes of Health panel said there's no evidence backing the use of convalescent plasma to treat coronavirus patients and that doctors should not treat it as a standard of care until more study has been done.
"There are insufficient data to recommend either for or against the use of convalescent plasma for the treatment of COVID-19," the panel of more than three dozen experts said in a statement posted on the NIH website Tuesday.
The statement, which was posted quietly, contradicts the Trump Administration's characterization of the treatment as "historic" and a "major advance" and directly refers to last week's emergency use authorization by the US Food and Drug Administration.
Convalescent plasma should not be considered standard of care for the treatment of patients with COVID-19," the committee, which evaluates treatments for coronavirus, said in the statement.
"Prospective, well-controlled, adequately powered randomized trials are needed to determine whether convalescent plasma is effective and safe for the treatment of COVID-19. Members of the public and health care providers are encouraged to participate in these prospective clinical trials."
Country / Region Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:
- Private group -

Recent Comments