You are here
 by - Nov 30 2014 - http://arstechnica.com
by - Nov 30 2014 - http://arstechnica.com
We know what antibodies stop it in its tracks—we now know where they attach.
The latest Ebola outbreak has dwarfed any that have occurred since the discovery of the virus in 1976; previous outbreaks have had lethality rates of up to 90 percent. Yet no vaccines or therapies are currently approved for human use, which limits our ability to treat patients and contain the outbreak. Mixtures of monoclonal antibodies (see sidebar) are a potential treatment option that has been used experimentally.
Monoclonal antibodies bind to a single structural feature on an infectious agent, such as the Ebola virus. These antibodies act as markers to flag down immune cells that destroy the foreign matter. Some antibodies can also be neutralizing, in that they block the harmful biological effects of a virus or prevent the budding of new virus particles. Mixtures of antibodies increase the treatment’s efficacy by limiting the opportunity of a mutant virus to escape recognition.
http://arstechnica.com/science/2014/11/finding-the-ebola-virus-vulnerable-points/



Comments
Research - Finding the Ebola virus’ vulnerable points
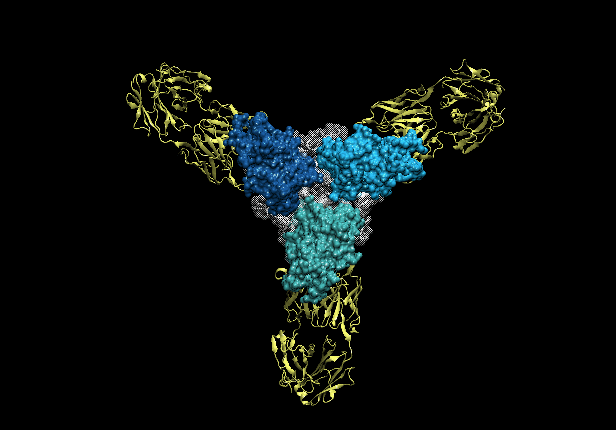
Structures of protective antibodies reveal sites of vulnerability on Ebola virus
pnas.org - Charles D. Murin, Published online before print November 17, 2014, doi: 10.1073/pnas.1414164111
PNAS December 2, 2014 vol. 111 no. 48 17182-17187
Abstract
Ebola virus (EBOV) and related filoviruses cause severe hemorrhagic fever, with up to 90% lethality, and no treatments are approved for human use. Multiple recent outbreaks of EBOV and the likelihood of future human exposure highlight the need for pre- and postexposure treatments. Monoclonal antibody (mAb) cocktails are particularly attractive candidates due to their proven postexposure efficacy in nonhuman primate models of EBOV infection. Two candidate cocktails, MB-003 and ZMAb, have been extensively evaluated in both in vitro and in vivo studies. Recently, these two therapeutics have been combined into a new cocktail named ZMapp, which showed increased efficacy and has been given compassionately to some human patients. Epitope information and mechanism of action are currently unknown for most of the component mAbs. Here we provide single-particle EM reconstructions of every mAb in the ZMapp cocktail, as well as additional antibodies from MB-003 and ZMAb. Our results illuminate key and recurring sites of vulnerability on the EBOV glycoprotein and provide a structural rationale for the efficacy of ZMapp. Interestingly, two of its components recognize overlapping epitopes and compete with each other for binding. Going forward, this work now provides a basis for strategic selection of next-generation antibody cocktails against Ebola and related viruses and a model for predicting the impact of ZMapp on potential escape mutations in ongoing or future Ebola outbreaks.
(CLICK HERE - RESEARCH - Structures of protective antibodies reveal sites of vulnerability on Ebola virus)